- Number Of Electrons In Calcium Ion
- Total Number Of Electrons In Calcium
- How Many Electrons For Calcium
- Number Of Electrons In Calcium
- Number Of Electrons In Calcium 40
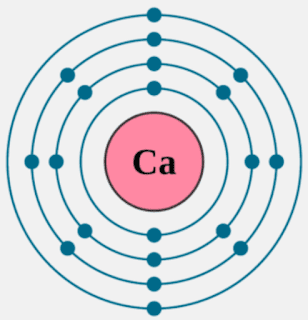
Calcium is an alkaline earth metal and present in the s-block of the periodic table. Calcium lies in 2nd group and 4th period of the periodic table. The electronic configuration of calcium is 4s2. The number of electrons present per shell in a calcium atom is 2, 8, 8, 2. Calcium has two electrons in. In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Calcium (Ca). From the Period. Calcium atoms have 20 electrons and 20 protons. There are 2 valence electrons in the outer shell. Calcium is an important element for life on Earth and is the fifth most abundant element in the Earth's crust. Under standard conditions calcium is a shiny, silvery metal. Calcium has 2 valence electrons. Calcium has 20 electrons so they are arranged 2. 2 Using orbital notation the electron structure is: 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)4s^(2) You can see that there are 2 electrons in the outer energy level and these are the valence electrons.
- Anonymous (age 14)
L.A. school district
Every atom basically has an infinite number of shells. The thing is that almost all of those shells are empty (they don’t have electrons in them). Electrons generally go into the orbital with the 'lowest energy.'
The first orbital that fills up is called the 1S orbital. This one can hold 2 electrons. Once you get to the third electron, you have to put it in the next orbital. This one is called the 2S orbital. (All 'S' orbitals hold 2 electrons.) Next comes the 2P shell. 'P' orbitals hold 6 electrons. After 2P is 3S, 3P, 4S, 3D, 4P, etc. ('D' orbitals hold 10 electrons each.) The order gets pretty complicated once you get this far, but you probably won’t need to know them much past 3P.
If your teacher is trying to make this less confusing, they may just pay attention to the numbers, not the letters. In this case you can just add them up. Shell 1 (1S) holds 2 electrons. Shell 2 (2S & 2P) and Shell 3 (3S & 3P) each hold 8 electrons. After that, the next 2 hold 18 each, then the next 2 hold 32. So far, scientists haven’t discovered any elements that use more orbitals than this.
As for 'how many shells the atom has,' I already said that it has an infinite number of empty ones. But the number of full ones is probably closer to what you’re looking for. For example, lets look at Calcium. Calcium has 20 electrons. (You can see this by looking at the atomic number of Ca on a .) The first 2 go in shell 1, leaving 18 more. 8 more go into shell 2, then 8 in shell 3. Then there are 2 left. This isn’t enough to fill the next shell, which would require 18 more, so we say that Calcium has 3 filled orbitals and 2 free electrons.
If an atom is what is called an 'ion,' then that means it has a different number of electrons than you’d expect. This is written by using little numbers with + or - after the atom's letters. Because electrons actually have a negative charge, a - sign means that the atom has more electrons than you’d think. For example, let’s take Calcium again. Like we said, Ca has 20 electrons. But Ca- has 21, and Ca2- has 22. Going the opposite way, Ca+ has 19, and Ca2+ has 18. (Since 18 makes exactly 3 filled orbitals, Ca2+ is Calcium’s most common ion.)
Ok..going back to what I said about electrons going to the orbital with the 'lowest energy.' What if you were to give those electrons some more energy? Lets say we take Helium, which has 2 electrons. These 2 electrons will be just enough to fill Shell 1. If I give them some more energy, one of those electrons jumps up to Shell 2. So there’s 1 electron in Shell 1 and 1 in Shell 2. But as soon as it can, this electron will fall back to Shell 1 again. When it does, it will release energy as light.
Hope this answers your question!
-Tamara
(published on 10/22/2007)
Follow-Up #1: atomic shells
- Thurein (age 17)
Kingston, Jamaica
For the 6 2P electrons, there are as you say 3 opposite-spin pairs of electrons, with the two electrons in each pair having the same ’orbit’, i.e. the same shape of cloud. The clouds in this case aren’t spherical but rather have lobes. The three different 2P orbital states differ in that their lobes point different directions.
Number Of Electrons In Calcium Ion
Mike W.
(published on 10/22/2007)
Follow-Up #2: electrons and energy
I don’t know if my question makes sense.. But please do reply me, thanks.
- Emma
Canada
Mike W.
(published on 10/22/2007)
Follow-Up #3: orbital existentials
- Azeem Notta (age 19)
Toronto, Canada
Mike W.
thanks, Inga
(published on 05/16/2013)
Follow-Up #4: orbital misery
- Devin (age 14)
Clovis CA
Yeah, that orbital stuff is complicated. I'm not sure why it gets introduced so soon in school. There would be a lot of much clearer things to learn.
Just for a little start on orbitals, since something about them was probably assigned, you might look at these pictures of some different orbital shapes that the electron cloud can take: . Orbitals have stable shapes that don't change in time. Other states do change in time.
As for your wishes for science, perhaps providing governments with nuclear weapons was a step in that direction. If they are used, however, there will be collateral damage.
Mike W.
(published on 09/19/2013)
Follow-Up #5: how many electrons per shell?
- Taylor (age 14)
UK
If each shell represented a spherically symmetrical cloud form ('S state'), you'd be right that each would hold only two electrons, given the two spin states. However, there are states that are not spherically symmetric. One way of expressing the P states has two lobes of cloud, with opposite sign of wave in each lobe. There are three independent ways of aligning those lobes, e.g. along x,y, or z axes. By symmetry these all have the same energy. For D states there are more lobes and five independent forms. In the conventional shell language, states that have almost the same energy, such as the lowest energy P states and the second-lowest-energy S states are lumped into the same shell, giving even more states per shell.
Mike W. Paradigm usb devices driver download for windows 10.
(published on 07/06/2016)
Follow-Up #6: why do atoms bond?
Total Number Of Electrons In Calcium
- Noah (age 14)
Winnetka, California, USA
Have look at follow-up 1 in this old thread. Maybe it answers your question.
https://van.physics.illinois.edu/qa/listing.php?id=523 https://van.physics.illinois.edu/qa/listing.php?id=523
Mike W.
(published on 04/10/2018)
Follow-up on this answer.
Click to see full answer
Then, why is a calcium ion smaller than a calcium atom?
Because the calcium ion donates two electrons to achieve the stable state. Thus, the radius of a calcium ion is smaller than a calcium atom.
Secondly, how does a calcium atom become an ion? When it loses electron(s) it becomes positively charged and is called a cation. Calcium atom with electron arrangement K (2),L(8),M(8),N(2) loses two electrons from its outermost shell ( N shell) and forms positive ions called Calcium , Ca2+ ion.
How Many Electrons For Calcium
Then, what does calcium ion do?
Number Of Electrons In Calcium
Calcium in biology. Calcium ions (Ca2+) contribute to the physiology and biochemistry of organisms cell. They play an important role in signal transduction pathways, where they act as a second messenger, in neurotransmitter release from neurons, in contraction of all muscle cell types, and in fertilization.
What does a calcium atom look like?
Number Of Electrons In Calcium 40
Calcium atoms have 20 electrons and 20 protons. There are 2 valence electrons in the outer shell. Calcium is an important element for life on Earth and is the fifth most abundant element in the Earth's crust. Under standard conditions calcium is a shiny, silvery metal. Ntt card reader driver.
