Seven metastable isotopes have been characterized; 68m Cu is the longest-lived with a half-life of 3.8 minutes. Isotopes with a mass number above 64 decay by β −, whereas those with a mass number below 64 decay by β +. 64 Cu, which has a half-life of 12.7 hours, decays both ways. Atomic Mass of Copper Atomic mass of Copper is 63.546 u.
Atomic Weight
The atomic weight is the mass of an atom, typically expressed in atomic mass units (amu). For an isotope, it is the mass of the nucleus, that is the mass of the protons and neutrons, as the mass of the electrons are considered negligible. In their natural state only 21 elements exist as single isotopes, that is a sample has nuclei of only one isotope, and these are called the mononuclidic elements. Most elements exist as a mixture of nuclei from multiple isotopes, and these are labeled as the polynuclidic elements. The atomic weight of a monuclidic element is that mass of that nuclide.
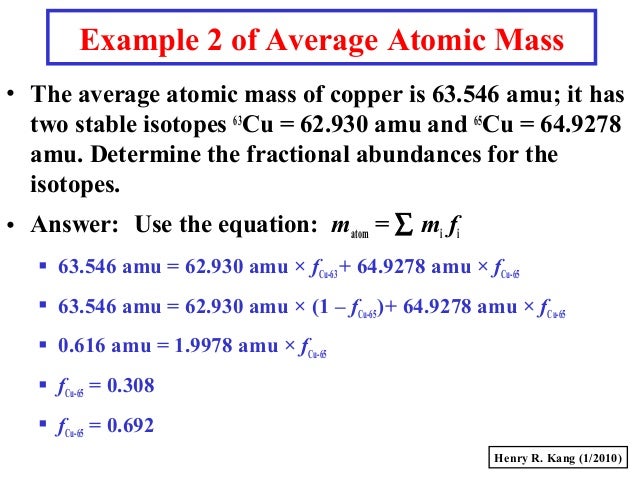
For a polynuclidic element the atomic weight is the average weight based on the fractional abundance of each isotope, and this is the value given on the periodic table. Copper has two isotopes, 63Cu (69.15%, mass=62.9300 amu) and 65Cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a single atom that weighs 63.55 amu.
| [underbrace{0.6915}_{fraction ; ^{63}Cu}underbrace{(62.9300, amu)}_{mass ; ^{63}Cu} + underbrace{ 0.3085}_{fraction ; ^{65}Cu} underbrace{(64.928 ,amu)}_{mass ; ^{65}Cu} = underbrace{63.55, amu}_{text{average mass}} note: ; 0.6915 + 0.3085 = 1] |
Rapoo input devices driver download. Figure (PageIndex{1}): Natural samples of copper contain two isotopes, and its atomic weight is to four significant digits is 63.55 amu, even though there is not a single atom of copper that weights 63.55 amu.
Exercise (PageIndex{1})
The atomic weight of chorine is ______________and the atomic number of chlorine-35 is________________.
- 35, 17
- 17, 35
- 35.4527; 17
- 35.4527; 35
C) the atomic weight is the average of mass of all isotopes of chlorine atoms and found below the symbol on the periodic table. The atomic number is the number of protons in all chlorine atoms and is found on the top of the symbol in the periodic table.
Atomic Mass

Atomic mass is based on a relative scale and the mass of 12C (carbon twelve) is defined as 12 amu.
Why do we specify 12C? We do not simply state the the mass of a C atom is 12 amu because elements exist as a variety of isotopes.
Carbon exists as two major isotopes, 12C, and 13C (14C exists and has a half life of 5730 y, 10C and 11C also exist; their half lives are 19.45 min and 20.3 days respectively). Each carbon atom has the same number of protons and electrons, 6. 12C has 6 neutrons, 13C has 7 neutrons, and 14C has 8 neutrons and so on. Since there are a variety of carbon isotopes we must specify which C atom defines the scale.
All the masses of the elements are determined relative to 12C.
By the way, the mass of an element is not equal to the sum of the masses of the subatomic particles of which the element is made!
Average Atomic Mass
Since many elements have a number of isotopes, and since chemists rarely work with one atom at a time, chemists use average atomic mass.
On the periodic table the mass of carbon is reported as 12.01 amu. This is the average atomic mass of carbon. No single carbon atom has a mass of 12.01 amu, but in a handful of C atoms the average mass of the carbon atoms is 12.01 amu.
Why 12.01 amu?
If a sample of carbon was placed in amass spectrometer the spectrometer would detect two different C atoms, 12C and 13C.
The natural abundances of 14C, 10C and 11C are so low that most mass spectrometers cannot detect the effect these isotopes have on the average mass. 14C dating is accomplished by measuring the radioactivity of a sample, not by actually counting the number of 14C atoms.
The average mass of a carbon is calculated from the information the mass spectrometer collects.
The mass spectrometer reports that there are two isotopes of carbon,
98.99% of the sample has a mass of 12 amu (not a surprise since this is the atom on which the scale is based).1.11% of the sample has a mass of 13.003355 amu (this isotope is 1.0836129 times as massive as 12C) Download optic dual-touch team input devices driver.
The average mass is simply a weighted average.
ave. mass = 12.01 amu
(Yes, the number 12.01 has the right number of significant figures, even though 1.11% only has 3 significant figures.)
If we know the natural abundance (the natural abundance of an isotope of an element is the percent of that isotope as it occurs in a sample on earth) of all the isotopes and the mass of all the isotopes we can find the average atomic mass. The average atomic mass is simply a weighted average of the masses of all the isotopes.
(Yes, the sig figs are correct.)
Another kind of question could be asked..
Copper has two isotopes 63Cu and 65Cu. The atomic mass of copper is 63.54. The atomic masses of 63Cu and 65Cu are 62.9296 and 64.9278 amu respectively; what is the natural abundance of each isotope?
substituting gives
One equation and two unknowns..is there another equation? If there is another equation we would have two equations and two unknowns, and a system of two equations and two unknowns is solvable.
Since there are only two major isotopes of Cu we know that
or
(eq. B)
Use eq. B to substitute for %63Cu in eq. A.
To the correct number of significant figures
Of course, a question like the one above could be turned around another way.
Gallium, atomic mass 69.72 amu, has two major isotopes, 69Ga, atomic mass 68.9257 amu, and 71Ga. If the natural abundance of each isotope is 60.00 and 40.00 % respectively what is the mass (in amu) of 71Ga.
The mole
Atomic Mass Of Cuo
What is the relative mass of 1 C atom as compared to 1 H atom?
What is the relative mass of 100 C atoms as compared to 100 H atoms?
What is the relative mass of 1 W atom as compared to 1 H atom?
What is the relative mass of 100 W atoms as compared to 100 H atoms?
The point here? As long as the number of atoms remains the same the relative mass does not change.
Atoms are small, and it is possible to place 1.0079 g of H on a balance (possible but not easy in the case of hydrogen).
It is also possible to place 183.9 g W, or 12.01 g of C on a balance.
Now, I state with absolute certainty that I have placed the same number of atoms on each balance! How do I know? I know because the relative masses of the samples on the balance, are the same as the relative masses of the individual atoms.
W:H = (183.9 g/1.0079 g):1 = 182:1C:H = (12.01 g/1.0079 g):1 = 11.92:1
Download pam bv usb devices driver. The number of atoms I placed on the balance is know as a mole.
Atomic Mass Of Cucl2
For many years the number of atoms in a mole remained unknown; however, now it is know that a mole of atoms contains 6.02214 x 1023 atoms.
So, the periodic table provides us with a great deal of information.
The periodic table lists
How To Calculate Atomic Weight
the mass of an atom in amu,the mass of a mole of atoms (i.e. the molar mass) in grams,
Atomic Mass Of Cuso4
and the mass of 6.02214 x 1023 atoms in grams
The atomic mass of C is 12.01 amu. What is the mass of 1 C atom?